|
|
Original Article
| ||||||
| Chemical composition, anthelmintic and antimicrobial activity of chloroform fraction of ethanol extract of Cleome rutidosperma | ||||||
| Frank N. I. Morah1, Gloria C. Apebende2 | ||||||
|
1PhD, Professor of Natural Products Chemistry, Chemistry Department, University of Calabar, Calabar, Cross River State, Nigeria 2MSc, Lecturer, Chemistry Department, University of Calabar, Calabar, Cross River State, Nigeria | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar articles in PubMed] [Similar articles in Google Scholar] |
| How to cite this article |
| Morah FNI, Apebende GC. Chemical composition, anthelmintic and antimicrobial activity of chloroform fraction of ethanol extract of Cleome rutidosperma. Edorium J Public Health 2018;5:100019P16FM2018. |
|
ABSTRACT
| ||||||
|
Aims: To determine the chemical constituents, antimicrobial and anthelmintic activities of chloroform fraction of the ethanol extract of the aerial part of Cleome rutidosperma. Methods: The powdered plant material was defatted with petroleum spirit and the residue extracted with ethanol. The ethanol extract was partitioned between water and chloroform to get the chloroform fraction whose chemical constituents were determined through GC-MS analysis. The antimicrobial sensitivity test was carried out using agar disc diffusion method while broth dilution method was used to determine the minimum inhibitory, minimum bactericidal and minimum fungicidal concentrations. 1%, 5% and 10% aqueous solutions of the extract were used for determination of anthelmintic activity against adult Lumbricus terrestris. Results: The present work shows that the chloroform fraction contains ten compounds which are being identified for the first time in Cleome rutidosperma. It also shows that the chloroform fraction has greater activity against gram positive bacteria. The present study, further shows that, the chloroform fraction of the ethanol extract has a strong anthelmintic activity. Conclusion: The chloroform fraction of Cleome rutidosperma ethanol extract contains ten natural products which have both antimicrobial and anthelmintic activities. Keywords: Antimicrobial activity, Anthelmintic activity, Cleome rutidosperma | ||||||
|
INTRODUCTION
| ||||||
|
Cleome rutidosperma is an African medicinal plant which has naturalized in Tropical America and Asia. It is an annual herb. Traditionally, in Nigeria, different parts of the plant are used for the treatment of paralysis, epilepsy, convulsions, spasm, earache and skin diseases especially fungal infections. Aqueous extract of the root has been shown to have anthelmintic activity against Haemonchus contortus [1]. It has antidepressant activity [2]. Its ethyl acetate extract has been shown to be an antibacterial and bio enhancing agent against multidrug resistant clinical isolates [3]. It has diuretic and antimicrobial activity [4], [5], wound healing activity [6], antimicrobial activity [7] , anticonvulsant activity [8], anti-arthritic activity [9], anti-diabetic activity [10] and antifungal activity [11] . The leaf has bitter taste like mustard and is employed in soup making [12]. Cleome rutidosperma has been employed in herbal medicine over the years but there is very scanty information on its chemical constituents. With development of resistance of different microorganisms to conventional drugs used in orthodox medical practice, the use of medicinal plants to control infections is increasing in popularity [13]. These herbal drugs have the added advantage of being cheap, readily available and environmentally friendly. The present work is therefore focused on identification of chemical constituents and antimicrobial and anthelmintic activities of the chloroform fraction of the ethanol extract of Cleome rutidosperma. | ||||||
|
MATERIALS AND METHODS
| ||||||
|
The aerial part of Cleome rutidosperma was harvested from Betem in Biase Local Government Area of Cross River State, Nigeria. It was authenticated by staff of the Herbarium unit, Botany Department, University of Calabar, Calabar. Voucher specimen of the plant was deposited in the herbarium for future reference. The plant material was air-dried for one week and ground to obtain a powder. 30g of the powdered plant material was defatted with petroleum spirit (60-80oC) for 2 hour in a Soxhlet extractor. The residue was further extracted with ethanol. This was distilled down over a steam bath to give a syrupy ethanol extract. The extract was partitioned between chloroform and water. The lower chloroform layer was collected, dried over anhydrous sodium sulfate and distilled down over a steam bath to give the chloroform fraction of the ethanol extract. The constituents of the chloroform fraction were identified through Gas chromatography-mass spectrometry (GC-MS) analysis using Agilent Hewlett Packard (7980A) with triple detector equipped with auto-injector (10μm syringe). Helium was used as the carrier gas. The column length is 30 cm, internal diameter 0.25μm, thickness 250 μm, treated with phenylmethylsiloxane. Ion source temperature is 250oC, pressure 16.2Psi, 1μm injector in split mode with split ratio of 1.50, with injection temperature of 300oC. The column temperature was raised at 35oC for 5min and changed to 150oC at the rate of 20oC min-1 and held for 5min before ionization. Microsoft solution software provided by the supplier was used to control the system and to acquire the data. Identification of the compounds was carried out by comparing the mass spectra obtained with those of the standard mass spectra from National Institute of Standard and Technology, NIST. Antimicrobial susceptibility test was done using agar disc diffusion method. The microorganisms used are Escherichia coli (gram negative), Streptococcus faecalis (gram -ve), Streptococcus aureus (gram +ve) and the fungi Aspergillus niger and Candida albicans. All these microbes are clinical isolates obtained from the Pathology Department, University of Calabar Teaching Hospital. The microbes were cultured and maintained using Cruicksharak method [14]. A stock solution of the chloroform fraction in water containing 100 μgcm-3 was prepared. Part of this was diluted with distilled water to get solutions containing 6.25, 12.5, 25, 50 and 100 μgcm-3. Mueller Hinton agar was used for both the bacteria and fungi tests. Filter paper discs were sterilized and separately soaked in solutions containing the different levels of the extract. They were placed in different plates containing the different test organisms. They were incubated at 37oC for 24h for bacteria and 48h for fungi. The zone of inhibition was recorded for each plate after incubation. 100mg of doxycycline was dissolved in 1000 cm3 of distilled water to give a 100μgcm-3 solution. This was diluted to get a 10 μgcm-3 solution of doxycycline which was used as the control. For the determination of minimum inhibitory concentration, MIC, 50, 25, 12.5, 6.25 and 3.13 μgcm-3 of the chloroform fraction in distilled water were placed in different test tubes and 1 cm-3 of water was added to each of them. Peptone water (Muller Hilton broth) 4 cm3 was added followed by addition of 24h-broth culture of the organism. The test tubes were sealed with sterile corks and incubated at 37oC for 24h. The test tubes were finally observed for clearance or turbidity. The first test tube with highest degree of clearance is taken as the minimum inhibitory concentration, MIC, while the one preceding it is taken as minimum bactericidal concentration, MBC, for bacteria or minimum fungicidal concentration, MFC, for fungi. This procedure was separately carried out for Escherichia coli, Candida albicans, Aspergillus niger, Streptococcus faecalis and Staphylococcus aureus. Adult Lumbricus terrestris was used for the anthelmintic test. They were collected few hours before test from decaying plantain stems. 1, 5 and 10% solutions of the extract in distilled water were prepared. Four preciously sterilized petri-dishes for each of the three levels of extract in water and a control were used. 25cm3 of each of the solutions is placed in a petri-dish. Also 25cm3 of phosphate buffer saline in a petri-dish served as the control. Five adult worms were placed in each of the four petri-dishes and observed at room temperature for 3h. The non-motile (dead) worms were counted and the percentage mortality calculated. | ||||||
RESULTS | ||||||
|
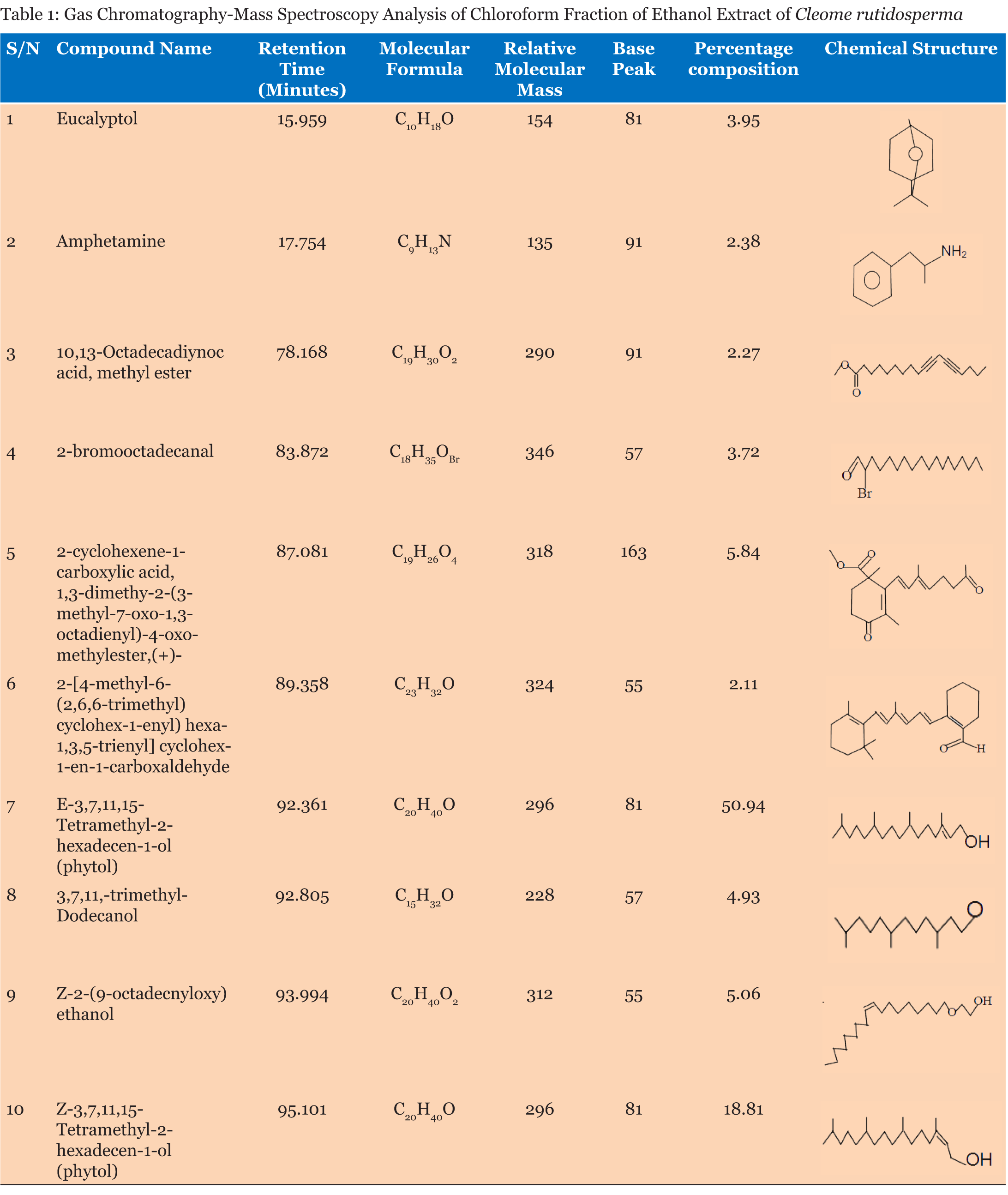
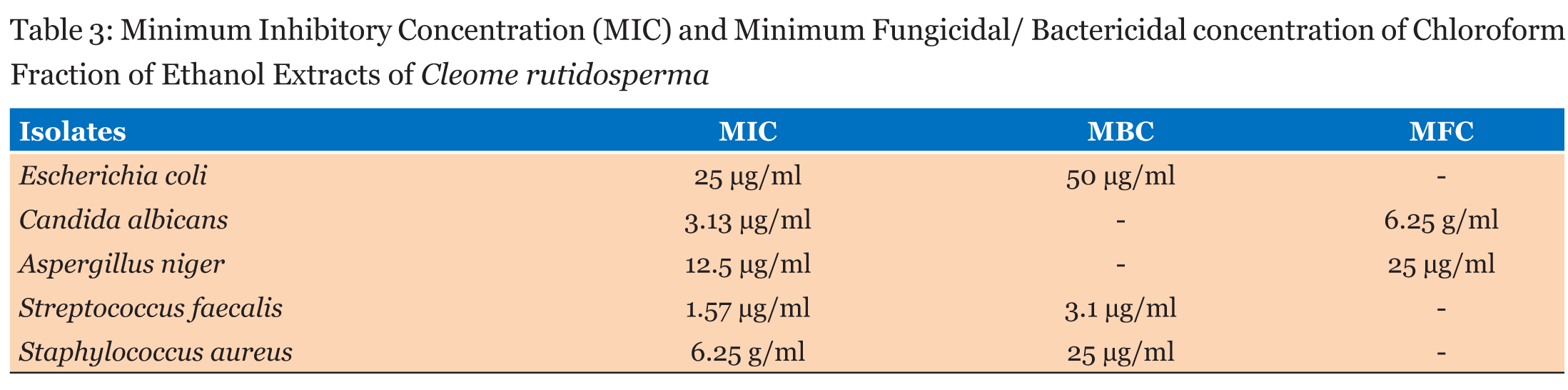
Table 1 gives the chemical composition of the chloroform fraction of the ethanol extract of Cleome rutidosperma aerial part. It contains ten identifiable organic compounds. With exception of amphetamine, the remaining nine compounds were oxygenated. Table 2 shows the antimicrobial sensitivity of the selected microbial pathogens whose growth was inhibited, to different extents, by the extract. The minimum inhibitory, minimum bactericidal and minimum fungicidal concentrations of the extract are given in Table 3 while Table 4 shows the effect of the extract on mortality of adult Lumbricus terrestris. | ||||||
DISCUSSION | ||||||
|
Ten compounds were identified in the chloroform fraction of the ethanol extract of Cleome rutidosperma. To the best of our knowledge there is no earlier report on the occurrence of these compounds in Cleome rutidosperma. Phytols constitute a total of 69.75% of the chloroform fraction and it is an antifungal and antimalarial agent which is very active against Salmonella typhi. It is also known to have antiulcer, antioxidant, anti-inflammatory and diuretic properties [15]. Eucalyptol constitutes about 3.95% of the chloroform fraction and it has strong antimicrobial and antifungal activity [16]. Amphetamine which constitutes about 2.38% of the chloroform fraction is a potent central nervous system (CNS) stimulant used in the treatment of obesity, narcolepsy and attention deficit hyperactivity, (ADHD) [17]. Table 2 shows that the chloroform fraction of the ethanol extract has inhibitory effect on the selected microorganisms. Table 3 shows that the minimal inhibitory concentration is 25 μgcm-3 for the gram negative Escherichia coli while it is 1.57 and 6.25 μgcm-3 for the gram positive Staphylococcus aureus and Streptococcus faecalis respectively. This implies that the selected gram positive bacteria are more sensitive to the chloroform fraction than the gram negative one. Table 3 also shows that the chloroform fraction has pronounced antifungal properties. The chemical constituents of the chloroform fraction are responsible for its biological activities. Both phytol and eucalyptol are mainly responsible for the observed antifungal and antimicrobial activities. Although these two compounds are held responsible for such biological activities, it is well known that biological activities of such major plant constituents are modulated by the minor constituent [18]. Earthworms show anatomical and physiological resemblance with intestinal round worm parasites of human beings. Because of their easy availability they are used as suitable models for screening of anthelmintic drugs [19]. This guided the choice of Lumbricus terrestris for this study. The result of anthelmintic test shows that all the adult worms died within 3 hour at 1.0% concentration while none died in the control showing that the chloroform fraction is quite efficacious as anthelmintic agent. The result is not surprising because an Indian species, Cleome icosandra, is known to have anthelmintic activity [20] and studies also show that Cleome viscose has dose dependent anthelmintic activity [21]. | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
CONCLUSION
| ||||||
|
The Cleome rutidosperma chloroform fraction of the ethanol extract contains ten natural products. These are identified for the first time in this plant species. It is more active against the gram positive than the gram negative bacteria. It is also an antifungal agent and a potent anthelmintic agent. This fraction will definitely serve as a useful drug. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Frank N. I. Morah – Substantial contribution to the conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of article, Revising it critically for important intellectual content, Final approval of the version to be published Gloria C. Apebende – Acquisition of data, Analysis and interpretation of data, Revising the article critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Consent Statement
Written informed consent was obtained from the patient for publication of this study. |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Frank N. I. Morah et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|